Background
HCM patients commonly suffer from diastolic dysfunction (also called heart failure with preserved ejection fraction (HFpEF), or left-sided heart failure): their hearts do not fill adequately. As a result, even after septal reduction therapy (SRT: myectomy or alcohol septal ablation ), some patients are quite symptomatic. Can mavacamten (Camzyos) help relieve their symptoms? Now a study ( Cremer et al. 2022 ) provides results on how mavacamten affects diastolic dysfunction among patients who have been referred for SRT.
Study design and goals
Researchers randomly assigned patients already on maximal doses of drugs like beta blockers or verapamil to either mavacamten or a placebo. These patients underwent both resting and stress echocardiograms, allowing the researchers to measure diastolic function. The researchers repeated the echos after 16 weeks. They asked several questions: How much did the patients’ diastolic function change? Which parameters from the echos related to changes in diastolic function? What proportion of patients improved in their grade of diastolic dysfunction?
Results
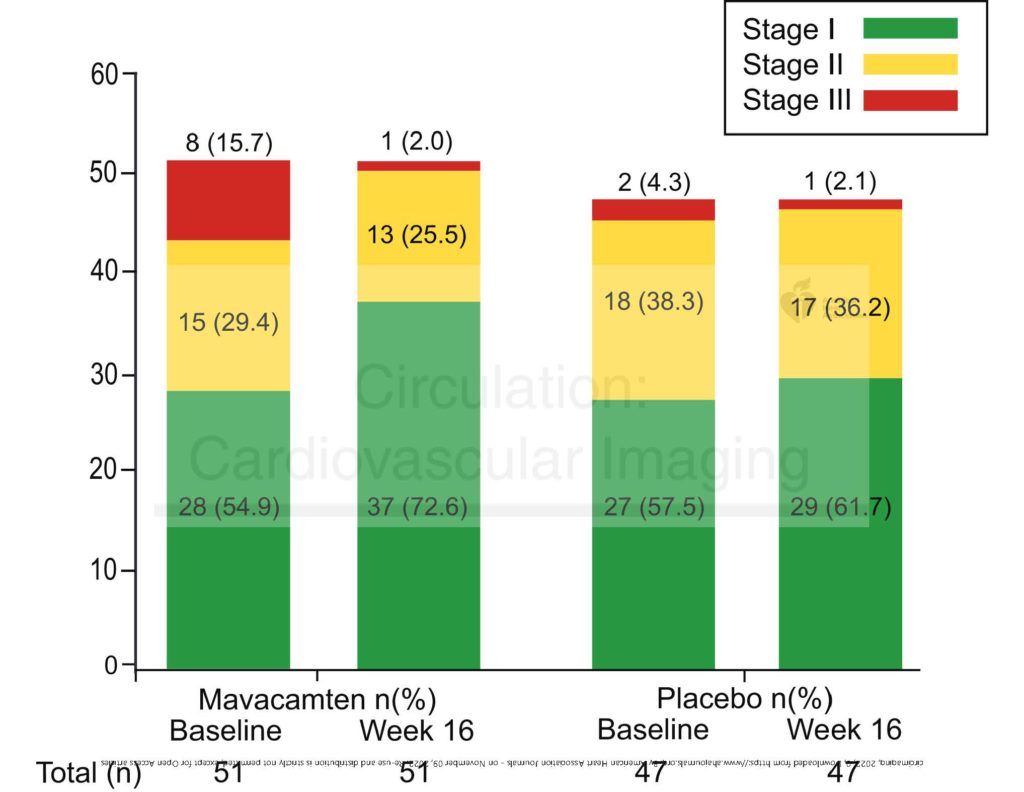
Fifteen of 51 patients (29%) receiving mavacamten showed improvement in their diastolic dysfunction grade. By contrast, 6 of 47 (13%) of patients in the placebo group improved. Thus mavacamten may have some efficacy in treating diastolic dysfunction. These improvements related to reductions in the E/e’ ratio (an index of the rate of filling in the left ventricle), and to the volume of the left atrium (LAVi) relative to body size. Importantly, the improvements did not depend on changes in the patients’ gradients or their mitral regurgitation.
Looking forward
The improvement in diastolic dysfunction grade is encouraging, suggesting new therapeutic approaches. However, the fact that improvement occurred in fewer than a third of the patients suggests that there is still much to be done in finding more effective treatments.
Literature cited
Cremer, P.C., Geske, J.B., Owens, A., Jaber, W.A., Harb, S.C., Saberi, S., Wang, A., Sherrid, M., Naidu, S.S., Schaff, H.V. and Smedira, N.G., 2022. Myosin Inhibition and Left Ventricular Diastolic Function in Patients with Obstructive Hypertrophic Cardiomyopathy Referred for Septal Reduction Therapy: Insights from the VALOR-HCM Study. Circulation: Cardiovascular Imaging. doi.org/10.1161/CIRCIMAGING.122.014986
The post Mavacamten Affects Diastolic Dysfunction appeared first on Hypertrophic Cardiomyopathy Association.


 Translate
Translate