An expert panel of HCM clinicians and researchers has published ( Brignole et al. 2022 ) a set of guidelines for diagnosing the causes of syncope (fainting) in HCM patients, and for treatment if indicated.
Fainting is fairly common among HCM patients (about 15%, Mascia et al. 2022 ), but its causes are usually unknown. Indeed, it is often assumed to be caused by arrhythmias, and used to make decisions about implanting ICDs. As there are many other things that can cause syncope, though, this can lead to inappropriate ICD implantation, as well as to failing to understand and treat the real cause of the syncope.
Causes of syncope
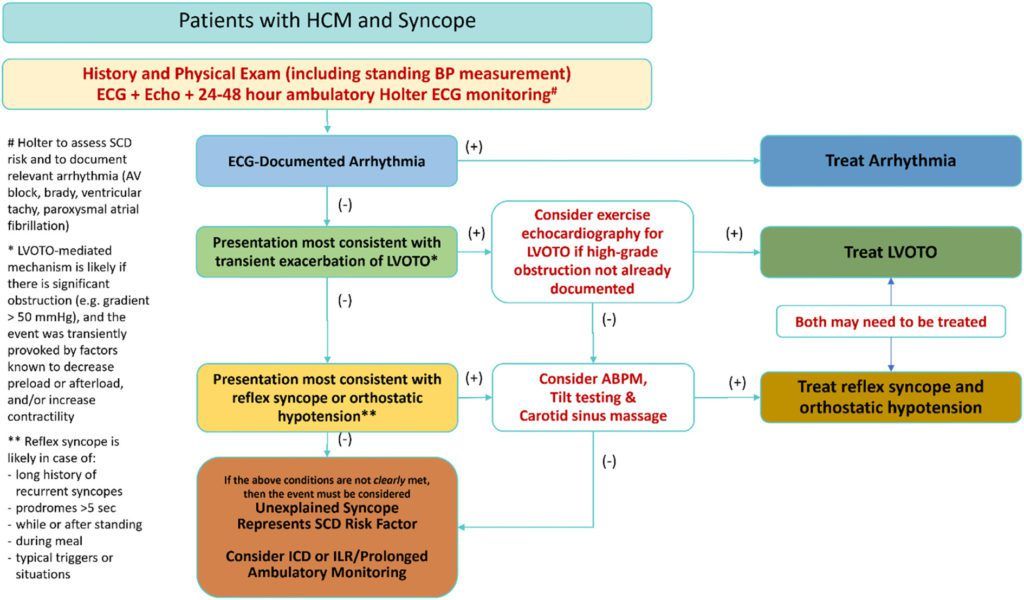
Most syncope (about 91%) among HCM patients appears to be unexplained, according to an earlier meta-analysis by the same panel ( Mascia et al. 2022 ). In addition to ventricular arrhythmias that can be prevented or aborted by ICDs, there are many other cardiac causes of fainting. There are also multiple causes of syncope that are neural in origin, as well as conditions that mimic syncope. Distinguishing among these is quite valuable clinically.
The recommended workup for HCM patients with syncope is
The guidelines are published in the International Journal of Cardiology.
Literature cited
Mascia, G., Crotti, L., Groppelli, A., Canepa, M., Merlo Andrea, C., Benenati, S., et al. Syncope in hypertrophic cardiomyopathy (part I): an updated systematic review and meta-analysis. 2022. Int. J. Cardiol., S973-983-7429, 10.1016/j.ijcard.2022.03.028
Brignole, M., Cecchi, F., Anastasakis, A., Crotti, L., Deharo, J.C., Elliott, P.M., Fedorowski, A., Kaski, J.P., Limongelli, G., Maron, M.S. and Olivotto, I., 2022. Syncope in hypertrophic cardiomyopathy (part II): An expert consensus statement on the diagnosis and management. International Journal of Cardiology. 10.1016/j.ijcard.2022.10.153
The post Expert consensus on syncope in HCM appeared first on Hypertrophic Cardiomyopathy Association.


 Translate
Translate